I. 总结
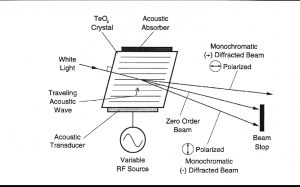
AOTF的原理是基于光在各向同性介质中的声衍射,该装置由一个压电传感器与一个双折射仪连接在一起构成,当传感器被应用的射频信号激活时,会在晶体中产生声波。传播声波产生了折射率的周期性调制,这提供了一个移动光栅,在适当的条件下,会衍射部分入射光束。对于固定声频,窄频段的光频满足匹配条件,并累积衍射。随着RF频率的变化,光带的中心也会相应地改变,从而保持相位匹配条件。
The near infrared region of the spectrum extends from 800nm to 2500nm. The absorption bands that are most prominent in this region are due to overtones and combinations of the fundamental vibrations active in the mid-infrared region. The energy transitions are between the ground state and the second or third excited vibrational states. Because higher energy transitions are successively less likely to occur, each overtone is successively weaker in intensity. The energy required to reach the second or third excited state is approximately twice or three times that needed for a first order transition. The wavelength of absorption is inversely proportional to the energy, therefore the absorption bands occur at about half and one third the wavelength of the fundamental absorption. In addition to the simple overtones, combination bands also occur. These usually involve a stretch plus one or more bending of rocking modes. Many different combinations are possible and therefore the NIR region is complex, with many band assignments unresolved.
Near Infrared Spectroscopy is currently being used as a quantitative tool which relies on chemometrics to develop calibrations relating a reference analysis of the constituent to that of the NIR optical spectrum. The mathematical treatment of NIR data includes Multi Linear Regression (MLR), Principle Component Analysis (PCA), Principle Component Regression
(PCR), Partial Least Squares (PLS) and discriminant analysis. All of these algorithms can be used singularly or in combination to yield the resultant goal of quantitative prediction and qualitative description of the constituents of interest in the sample.
- Methodology
In the manufacturing process of phtalic anhydride the solid anhydride is grated into flakes by a rotating drum. Water is used to cool the drum and due to reasons inherent to the process, penetration of water into the phtalic anhydride occurs. Some anhydride reacts to form phtalic acid when water penetrates. The time that elapses from the onset of the penetration until detection is critical because the product being processed during that period is likely to be discarded. A reliable and fast determination of the onset of such an event can be extremely useful from an economic point of view. The AOTF-NIR Luminar spectrometer can serve as a very fast, real-time, on-line detection system for such an occurrence. The phtalic anhydride has 2 C-O-C bonds in a pentagonal ring that disappear when it reacts with water. Two carboxylic groups are formed (HO-C=O) that give rise to absorption bands which do not naturally exist in the anhydride. Therefore, it is expected that phtalic acid should be detectable in low concentrations when mixed with the anhydride. It is possible that part or most of the water that penetrates will not react with the anhydride in a short time. However, it is expected that the 1950nm absorption band of the water will be detectable as soon as penetration occurs because it has a very large absorption coefficient.
A Brimrose AOTF-NIR Luminar Free Space spectrometer with a small area beam was used. The spectrometer is designed to operate between 1200nm and 2300nm. The scanning speed is 4000 wavelengths per second. 200 scans were collected and averaged into one spectrum for each data point. Acquisition time was about 14 seconds per spectrum. 21 samples were prepared by blending varying amounts of phtalic acid and phtalic anhydride in a blender. The samples ranged from pure phtalic anhydride to 2% by weight of phtalic acid in increments of 0.1%. The blended samples were placed in polyethylene bags and stored in a dry desiccator. The bags were opened and a sample was poured into a small petri dish shortly before spectra collection. The sample was placed on the spectrometer support surface at a fixed distance from the main lens and the collection of spectra was done through the glass. The same dish was used for all the samples to minimize interference. The spectra were collected in transmission mode and processed into absorbance.
III. Results
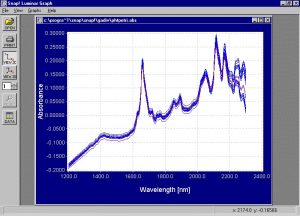
- Spectra
- Regressions and Modeling
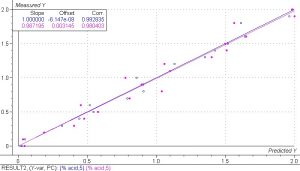
The PLS 1 regression model correlating phtalic acid in phtalic anhydride to spectral data showed excellent results, especially for such a small sample set. Past experience has shown that using a sample set of 100 or more samples significantly increases the accuracy and robustness of any model. The correlation coefficients are a measure of the correlation between the calibration and validation sets and the numbers of 0.993 and 0.980 for the calibration and validation sets show that this model can accurately predict phtalic acid in phtalic anhydride from spectral data.
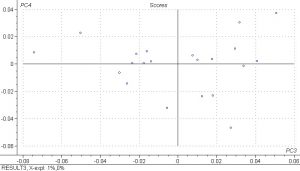
PCA analysis was done on the spectral data and the scores plots below show PC1 vs. PC2 and PC3 vs. PC4. Principle components are a measure of how many iterations the model must do to explain the data and 5 were done for the PLS model. The results show that there is fairly good separation between the samples and that the spectral differences are large.
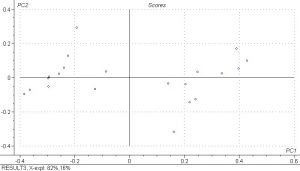
- Conclusions and Recommendations
The results of this study clearly prove that identification and quantitative determination of the presence of small quantities of phtalic acid in phtalic anhydride is feasible using spectral data obtained from the Brimrose AOTF-NIR Luminar Free Space spectrometer and a calibration model. The speed and insensitivity to mechanical vibrations of the spectrometer make it an ideal tool for a real-time on-line application for detection of the acid in the anhydride. Even if some of the penetrating water is unreacted, past experience has shown that the Brimrose AOTF-NIR spectrometer can easily detect changes in moisture and the same will hold true here. The results of the model were very good and they will be even better when a larger sample set is used for the calibration model. It is recommended to perform a test simulating the penetration of water in small quantities into anhydride. A method for conducting this test is to spray small quantities of water on the anhydride flakes. Spectra can be collected at varying time intervals. The consecutive spectra should show the progress of the acid level with exposure time. Several such samples should be prepared and they should all be sprayed with varying amounts of water. Quantitative analysis is not an absolute must for this application. Once a “normal” spectra is characterized by the PCA model, it is possible to define a “deviant” spectra that corresponds to a predetermined level of water, acid content, or a combination of both. These “deviant” conditions can be used to trigger alarms or be used for some other means of process control once the conditions are identified by the system. It is concluded that a Brimrose AOTF-NIR spectrometer can determine the presence of small quantities of phtalic acid in phtalic anhydride.